- Anasayfa
- Research and Laboratories
- Laboratories
- Corrosion Laboratory
Laboratories
- Laboratories
-
- Electronic Materials Laboratory
- Tribology Laboratory
- Casting Laboratory
- Heat Treatment Laboratory
- Chemistry Laboratory
- Corrosion Laboratory
- Ceramic Laboratory
- Metallography Laboratory
- X-Ray Diffraction Laboratory
- Mechanic Laboratory
- Plastic Forming Laboratory
- Advanced Materials Laboratory
- Characterization Laboratory
- Simulation Laboratory
- Specimen Preparation Laboratory
- Composite Laboratory
- Laboratory Service Working Systems
- Industrial Services

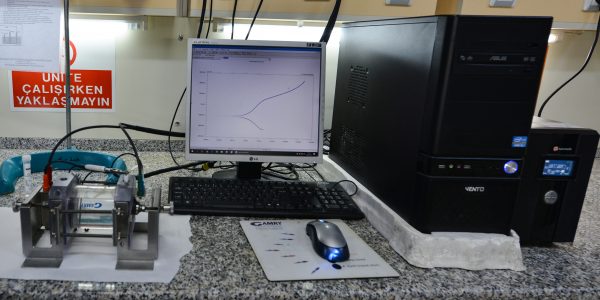
Corrosion Laboratory

Aim:
Corrosion, which leads to corruption of metals by electrochemical reactions, is an important factor that controls the design and maintenance of the facilities and equipment. Losses of about 4.5% of the gross national revenue are corrosion based and there is a need to be informed, conduct research and take precautions. The Corrosion Laboratory was founded for to give more information on the subject for research conducted at the undergraduate and graduate level. In addition, solving corrosion problems of industry and carrying out educational projects are also main reasons for founding the laboratory. .
Services
Analyses and Tests
- Measuring of the electrode potential, corrosion speed and polarization resistance of metals in different solutions,
- Examining the organic and inorganic coatings behavior, salt spraying test,
- Examining of pitting corrosions of metallic materials,
- Investigation of materials microstructures behavior of corrosion, material selection for corrosive environments,
- Determining the anodic and catholic polarization curves, measuring critical pitting corrosion potential and temperature,
- Determination of granular and inter-granular corrosion behavior of metals; dezincification test, corrosion in Weld Seams, and establish the grain boundary sensitivity,
- Electrolytic polishing, coating and measuring of the galvanic coatings thickness.
Analyzes Used in Scientific Research Activities
- Measuring of the electrode potential, corrosion speed and polarization resistance of metals in different solutions,
- Examining the organic and inorganic coatings behavior, salt spraying test,
- Examining of pitting corrosions of metallic materials,
- Investigation of materials microstructures behavior of corrosion, material selection for corrosive environments,
- Determining the anodic and catholic polarization curves, measuring critical pitting corrosion potential and temperature,
- Determination of granular and inter-granular corrosion behavior of metals; dezincification test, corrosion in Weld Seams, and establish the grain boundary sensitivity,
- Electrolytic polishing, coating and measuring of the galvanic coatings thickness.
Laboratory Infrastructure
- Electrochemical Test System
- Potential Drop Device
- Magnetic Stirrer and Heater
- Salt Spraying Test
- Water Purification Device
- Water Bath Test
- Precision BalanceDevice
Laboratory Images
Team
Contact
Department of Metallurgical and Materials Engineering